During Back Care Awareness Week, which this year is targeted at the Education sector, BackCare will be conducting media interviews and distributing promotional packs containing posters, pamphlets/leaflets and flyers.
Their aim is to disseminate guidance and advice on maintaining healthy backs for children during their school life and beyond.
BackCare have issued a press release, which you can download here.
Back Care Awareness Week runs 2 – 6 October 2017.
If you are interested in the information packs, you can get them here.…
Read more of this article



 Physical activity seems to have been the theme for the last few weeks. The latest phase of Public Health England’s ‘One You’ campaign
Physical activity seems to have been the theme for the last few weeks. The latest phase of Public Health England’s ‘One You’ campaign 
 NRAS are pleased to announce a FREE Rheumatoid Arthritis event in Solihull taking place on Saturday 16th September. The event takes place at The Village Hotel, Solihull (B90 4JG) and there is plenty of free parking available. Please arrive for refreshments at 13:30 with talks due to start at 13:45 and the event will run until approximately 16:00.
NRAS are pleased to announce a FREE Rheumatoid Arthritis event in Solihull taking place on Saturday 16th September. The event takes place at The Village Hotel, Solihull (B90 4JG) and there is plenty of free parking available. Please arrive for refreshments at 13:30 with talks due to start at 13:45 and the event will run until approximately 16:00.
 ARMA Stoke would like to let you know they are having their 3rd meeting of the year for the ARMA/Haywood user group.
ARMA Stoke would like to let you know they are having their 3rd meeting of the year for the ARMA/Haywood user group. 
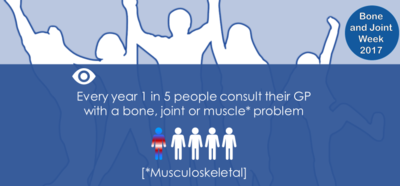 Bone and Joint Week 2017 runs from 12th-20th October
Bone and Joint Week 2017 runs from 12th-20th October




 BASEM is excited to launch its new partnership with OrthoEvidence (OE).
BASEM is excited to launch its new partnership with OrthoEvidence (OE).
 Join our webinar on Friday the 8th September at 12pm to find out.
Join our webinar on Friday the 8th September at 12pm to find out.