The government has announced a relaxation of the guidance to people in England who are clinically extremely vulnerable and therefore were advised to shield. (At the time of writing, guidance in the other nations had not changed). The chance of encountering coronavirus in the community has fallen: less than 1 in 1,700 are now estimated to have the virus, down from 1 in 500 four weeks ago. Whilst the relaxation is good news for many people who have gone months without leaving their houses, ARMA remains concerned that important protections are being lifted too soon.…
Read more of this article
Category: Arthritis News
Advanced Clinical Practice (ACP) MSK standards and framework
 Health Education England (HEE) in conjunction with the Musculoskeletal Association of Chartered Physiotherapists (MACP) has commissioned a piece of work to develop the operational aspect of the Advanced Clinical Practice (ACP) MSK standards.
Health Education England (HEE) in conjunction with the Musculoskeletal Association of Chartered Physiotherapists (MACP) has commissioned a piece of work to develop the operational aspect of the Advanced Clinical Practice (ACP) MSK standards.
SOMM has been selected to be part of the MSK partnership task & finish group that will be focussing on developing mechanisms of governance and quality assurance of MSK practice as part of the accreditation route.
This piece of work will be completed in tandem with the production of an ACP MSK standards document to be used by multi professionals working towards ACP accreditation via HEE.
New guidance on corticosteroids
 The guidance ‘Management of Patients with Musculoskeletal and Rheumatic Conditions on Corticosteroids’ has now been superseded by the following guidance, issued on 16 June 2020. It applies to:
The guidance ‘Management of Patients with Musculoskeletal and Rheumatic Conditions on Corticosteroids’ has now been superseded by the following guidance, issued on 16 June 2020. It applies to:
Management of patients with musculoskeletal and rheumatic conditions who:
- – are on corticosteroids
- – require initiation of oral/IV corticosteroids
- – require a corticosteroid injection
The new guidance can be read here [open PDF].
SOMM’s Injection Module Coordinator, Paul Hattam, has submitted his view on the guidance for corticosteroid injection that has been provided throughout the COVID-19 pandemic, as an open letter to the CSP’s ‘Frontline’ magazine.…
Read more of this article
Sport Rehabilitators supporting the NHS
 Sport Rehabilitators and Covid-19 – working with patients and supporting the NHS
Sport Rehabilitators and Covid-19 – working with patients and supporting the NHS
For an article on the Rehab Recruits scheme, BASRaT spoke to two sport rehabilitators to take a closer look at the fantastic work they are doing.
The NHS and MSKREFORM appealed for professionals who could temporarily work in an NHS role. The Rehab Recruits programme from MSKReform aimed to bring willing MSK clinicians together to help the NHS in its fight against Covid-19.
Sport Rehabilitators, Megan Thorley and Daniel Baker volunteered; read their stories here.
BOA – People waiting for orthopaedic surgery
 A British Orthopaedic Association message to people waiting for joint replacement and other orthopaedic surgery
A British Orthopaedic Association message to people waiting for joint replacement and other orthopaedic surgery
Growing waiting lists are affecting all planned surgery, but particularly orthopaedics which has the largest overall waiting list of any individual specialty. The BOA’s statement [published 26 June 2020] is a response to the concern, frustration and a lack of information available to people waiting for surgery. It explains how services can restart, gives advice on what to do, and provides resources to help people while they wait, as well as some FAQs.…
Read more of this article
Osteopaths now open for general practice in UK
 The Institute of Osteopathy can confirm that osteopaths operating in all areas of the UK can now open to general practice following the implementation of the relevant adaptations required to manage the risk of infection of COVID-19.
The Institute of Osteopathy can confirm that osteopaths operating in all areas of the UK can now open to general practice following the implementation of the relevant adaptations required to manage the risk of infection of COVID-19.
We know that following an extended period of lockdown by the general public, many people are experiencing issues with their MSK health, which can also have consequences for other areas of their health and well-being. The iO has issued an information video for the public so that they can be confident of the steps that will have been taken to put their safety first while accessing osteopathic care.…
Read more of this article
NRAS elects new Chair

Simon Collins
 NRAS is delighted to announce Simon Collins as the newly elected Chair of the Board of Trustees and would like to thank Gordon Taylor for four years as chair. The Society is pleased that Gordon will continue to serve as an NRAS Trustee.
NRAS is delighted to announce Simon Collins as the newly elected Chair of the Board of Trustees and would like to thank Gordon Taylor for four years as chair. The Society is pleased that Gordon will continue to serve as an NRAS Trustee.
Four newly elected Trustees have also joined the established board and they are Ellie Potter, Rich Flowerdew, Simone Webb and Lindsey Cook.…
Read more of this article
Good Boost and NRAS
 NRAS has been working with the social enterprise Good Boost, who champion the phrase “Move More, Have Fun, Feel Better.” Their mission is to create more affordable and accessible options for MSK community health services, and they use technology at the forefront for change.
NRAS has been working with the social enterprise Good Boost, who champion the phrase “Move More, Have Fun, Feel Better.” Their mission is to create more affordable and accessible options for MSK community health services, and they use technology at the forefront for change.
With growing numbers of physiotherapy and joint surgery appointments postponed due to Covid-19, their team of physiotherapists, osteopaths, MSK researchers, software engineers and AI specialists recognised the need for solutions providing personalised support at home.…
Read more of this article
What questions would you like to see answered by psoriatic arthritis research?
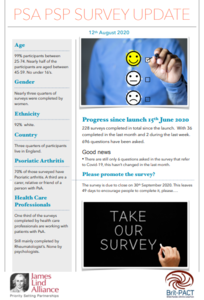 The British Psoriatic Arthritis Consortium is working with the James Lind Alliance to run a priority setting partnership (PSP). The aim is to create a national top-10 list of research priorities for psoriatic arthritis. They want ideas and thoughts from people with different experiences and backgrounds: those with psoriatic arthritis, their families, carers and clinicians.
The British Psoriatic Arthritis Consortium is working with the James Lind Alliance to run a priority setting partnership (PSP). The aim is to create a national top-10 list of research priorities for psoriatic arthritis. They want ideas and thoughts from people with different experiences and backgrounds: those with psoriatic arthritis, their families, carers and clinicians.
The survey has now been open for two months and 228 surveys have been completed. You can read information on who has completed the survey so far in the survey’s update document.…
Read more of this article
Changes to shielding guidance
The shielding guidance has changed so that from 1 June 2020 people who are shielding are advised that they may go outside once per day following strict social distancing. No other aspect of the guidance has changed, so people are still advised that they should remain 2 metres away from other members of their household, minimise the time spent in shared spaces, etc. Some people with MSK conditions are shielding, either because of the combination of medication they are taking or because they have other conditions that make them extremely vulnerable.…
Read more of this article









