 ARMA welcomes the 10 Year Health Plan and calls for a national MSK strategy to unlock its full potential
ARMA welcomes the 10 Year Health Plan and calls for a national MSK strategy to unlock its full potential
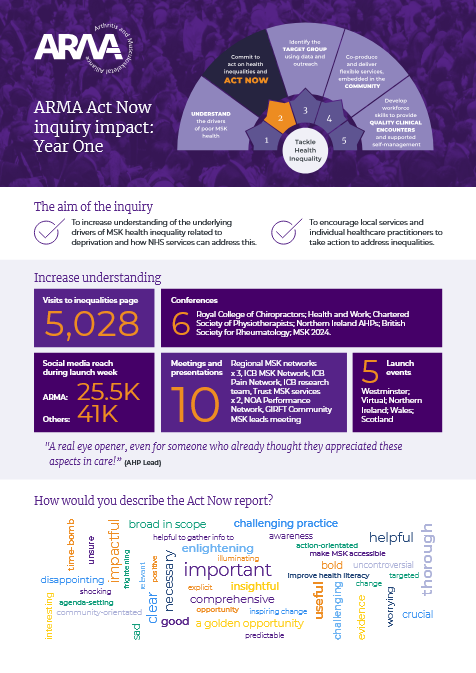
The Arthritis and Musculoskeletal Alliance (ARMA) welcomes the publication of the Government’s 10 Year Health Plan for England and its clear commitment to tackling widening health inequalities and focusing on healthy life expectancy. We are pleased to see many of the themes and recommendations from ARMA’s Act Now report reflected in the Plan, including a commitment to listening to the voice of patients and directing investment towards communities with entrenched health disparities.…
Read more of this article







 DEXA-scanner expansion to deliver 29,000 extra bone-density scans a year.
DEXA-scanner expansion to deliver 29,000 extra bone-density scans a year. Orthopaedic Research UK has launched this year’s Ronald Furlong Fund for startups solving unmet needs in MSK health.
Orthopaedic Research UK has launched this year’s Ronald Furlong Fund for startups solving unmet needs in MSK health.
