 Read the Summer-Autumn 2017 update newsletter from the Department of Rheumatology at Portsmouth Hospitals NHS Trust, and the Portsmouth ARMA Network, which features:
Read the Summer-Autumn 2017 update newsletter from the Department of Rheumatology at Portsmouth Hospitals NHS Trust, and the Portsmouth ARMA Network, which features:
- Events and conference details
- Flu and Pneumonia vaccination dates
- “No Time for Pain” Patient-Carer workshop
– discussing strategies for managing chronic and persistent pain associated to your Arthritis and Connective Tissue Disease with Partners/Family/Carers encouraged to attend as guests - “Tired of Being Tired” course
– a 7 week programme exploring different self-management and relaxation techniques to improve quality of life
Alongside the update are the fliers and forms related to upcoming events –
Day to Day Living with Arthritis and Connective Tissues Diseases Conference 2017:
Programme for healthcare professionals with registration form.…
Read more of this article




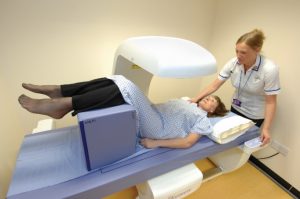 The National Osteoporosis Society delivers a biennial course popular with health professionals working in the field of musculoskeletal conditions, radiography, radiology and DXA.
The National Osteoporosis Society delivers a biennial course popular with health professionals working in the field of musculoskeletal conditions, radiography, radiology and DXA.
 Venue: Royal College of Physicians,
Venue: Royal College of Physicians, 


 The Edgar Stene Prize 2018 competition is open to people 18 years and over who are living with a Rheumatic and Musculoskeletal (RMD) disease. The 2018 prize will be awarded to the winning essay on the topic:
The Edgar Stene Prize 2018 competition is open to people 18 years and over who are living with a Rheumatic and Musculoskeletal (RMD) disease. The 2018 prize will be awarded to the winning essay on the topic:
 One thing I have been really struck by since starting this role is just how much musculoskeletal conditions impact across the breadth of a person’s life, and just how much unaddressed MSK conditions impact other parts of health services.
One thing I have been really struck by since starting this role is just how much musculoskeletal conditions impact across the breadth of a person’s life, and just how much unaddressed MSK conditions impact other parts of health services.
 The current delay to diagnosis of ankylosing spondylitis (AS) stands at 8.5 years. NASS are committed to reducing this delay to diagnose. There are many factors impacting on this delay but an important issue is the recognition of AS in primary care and prompt referral on to rheumatology.
The current delay to diagnosis of ankylosing spondylitis (AS) stands at 8.5 years. NASS are committed to reducing this delay to diagnose. There are many factors impacting on this delay but an important issue is the recognition of AS in primary care and prompt referral on to rheumatology. On 10th July 2017, NICE issued a new Technology Appraisal (TA) for bisphosphonates which links the recommendations for drug treatments to advice on fracture risk assessment outlined in their earlier guidance. The TA is not intended to provide treatment thresholds but offers recommendations on cost-effective use of bisphosphonates when using fracture risk assessment as described in their guideline
On 10th July 2017, NICE issued a new Technology Appraisal (TA) for bisphosphonates which links the recommendations for drug treatments to advice on fracture risk assessment outlined in their earlier guidance. The TA is not intended to provide treatment thresholds but offers recommendations on cost-effective use of bisphosphonates when using fracture risk assessment as described in their guideline 
 AMs in the National Assembly for Wales voted overwhelmingly in favour of a motion calling for a full multidisciplinary paediatric rheumatology service to be developed in Wales. Despite Cabinet Members abstaining, 27 AMs voted to support the motion, with nine abstentions and none against. There is now significant pressure on the government to take action.
AMs in the National Assembly for Wales voted overwhelmingly in favour of a motion calling for a full multidisciplinary paediatric rheumatology service to be developed in Wales. Despite Cabinet Members abstaining, 27 AMs voted to support the motion, with nine abstentions and none against. There is now significant pressure on the government to take action.